Read the instruction manual carefully before using the device,
especially the safety instructions,
and keep the instruction manual for
further use. If you pass on the device to third parties, be sure
to include this instruction manual.
GB IMPORT
Safety instructions
• This device must only be used for the purpose described in these instructions. The manufacturer
is not liable for damage resulting from improper use.
• Do not use the device in the presence of ammable anaesthetic mixtures with oxygen or nitrous
oxide (laughing gas).
• This device is only suitable for the anaesthesia and ventilation of the lungs.
• This device may only be used with the original accessories, which are listed in these instructions.
• Do not use the device, if you spot damage or you notice something unusual.
• Never open the device.
• This device consists of delicate components and must be handled with care. Observe the stor
-
age and operating conditions in the chapter “T
• Protect the device from: - water and moisture, - extreme temperatures, - knocks and dropping, -
dirt and dust, - strong sunlight, - heat and cold
• Adhere to the safety regulations applicable to electrical appliances, in particular the following:
- Never touch the device with wet or moist hands. - Position the device on a level and stable sur
-
face during use. - Do not pull the power cable or the device to remove the plug from the socket.
- The power cable plug is used to disconnect the device from the power supply
always remain accessible during use.
• Before connecting the device, make sure that the electrical data on the label on the bottom of the
device match the data of the mains.
• In case the mains plug of the device does not t into the socket, contact qualied personnel to re -
place the mains plug. In general, the use of adapters and extension cables is not recommended.
If it is essential to use them, then they must meet the safety regulations. In this case, however
permitted limit values, which are specied on the adapters and extension cables, must always be
adhered to.
• Do not leave the device plugged in when not in use; remove the plug from the socket when the
device is not being used.
• The installation must be carried out in accordance with the manufacturer
-
rect installation can cause damage to people, animals and objects, for which the manufacturer
cannot be held liable.
• Do not replace the charging cable of this device. In the case of a faulty cable, contact a technical
assistance centre approved by the manufacturer
• The charging cable should always be fully unwound to avoid dangerous overheating.
• Before every cleaning or maintenance operation, the device must be switched off and the power
cable removed from the socket.
• Only use the medicine prescribed to you by your doctor and follow the instructions of your doctor
regarding dosage, duration and frequency of the therapy
• Only use the parts specied by the doctor in accordance with your specic illness.
• Only use the nosepiece if expressly instructed to do so by your doctor
are NEVER inserted into the nose, but are only held as near as possible in front of the nose.
• Check on the package insert of the medicine, whether there are contraindications for use with the
usual systems for inhalation therapy
• When positioning the device, make sure that the On/Off switch can be easily reached.
• For reasons of hygiene, do not use the same accessories for more than one person.
• Do not tilt the nebulizer by more than 60°.
• Do not use the device near strong electromagnetic elds such as mobile phones or radio equip
-
ment. Keep a minimum distance of 3.3 m to such devices when using this device.
• Make sure that children do not use the device unsupervised; some parts are so small that they
could be swallowed. T
of tripping, they are not kinked and the risk of strangulation is eliminated.
• The use of this device is not a replacement for visiting the doctor
Intended use
The MEDISANA
vice is designed for the nebulization of liquids and liquid medicines (aerosols) and for the
treatment of the upper and lower airways.
Preparing the device
Before the rst use, we recommend cleaning all components - as described in the
chapter ”Cleaning and disinfection”.
In between, you can charge the device with the USB cable
w
and USB charging adapter
e
included. T
charging adapter and plug the other end of the cable into the charging socket
3
of the
inhaler
2
.
Application
Y
Charge the device as described in 'Preparing the device' (for example for use while travelling) or use the cable and
adapter as a direct power supply
T
1
.
While inhaling, sit upright and in a relaxed position at a table (not in an armchair),
so as not to compress the airways and therefore not to impair the effectiveness of the treatment. Do not lie down
during the inhalation. Stop the inhalation if you feel unwell.
After
1
in
order to switch off the device and remove the plug from the electrical socket.
Empty the remaining inhalation solution from the nebulizer and clean the device as described in the chapter “Cleaning
and disinfection”.
This device was developed for operation in 30 minutes On / 30 minutes Off mode. Please switch of
after 30 minutes and wait a further 30 minutes, before you continue the treatment.
• The device does not require calibration. Modication of the device is not permitted.
Scope of delivery
• 1 MEDISANA Inhaler IN 600/605 (
1
On/Off button,
2
Charging indicator
3
Micro-USB port (charging
socket),
4
Position of the air lter
5
Connection for the air tube)
• 1 Instruction manual
• Accessories:
6
Nosepiece,
7
8
Nebulizer
9
Mouthpiece,
0
q
Child face mask, IN 605: Baby face mask (without illustration)
w
Micro-USB cable (charging cable),
e
USB charging adapter
r
5 x
4
),
Storage bag (IN 605: storage pouch) without illustration.
Cleaning and disinfection
• Clean all accessories thoroughly after each treatment in order to remove residues of medicinal product and
possible impurities.
• Use a soft, dry cloth and a non-abrasive cleaning agent to clean the compressor
• Make sure that there is no intrusion of liquids into the device and the power cable is disconnected.
Cleaning and disinfection of the accessories
Follow the instructions for cleaning and disinfecting the accessories exactly
performance of the device and the success of the therapy
Before and after each application
1. T
8
anticlockwise in order to open the nebulizer and remove the atomiser
head.
2. W
9
and the nosepiece
6
. Then place in boiling water for 5
minutes.
3. W
4. Assemble the nebulizer parts again and connect the nebulizer to the air tube.
5. Switch the device on and leave in use for 10-15 minutes.
Use only cold sterilisation solutions in accordance with the manufacturer
Do not boil or autoclave masks and the air tube.
Maintenance and care
Replacement of the nebulizer
Replace the nebulizer
8
after a longer period of non-use, if it has deformations or cracks or if the atomiser
head is blocked by dried-up medicine, dust, etc. We recommend replacing the nebulizer after 6 to 12 months
depending on use. Only use the original nebulizer!
Replacement of the air lter
Under normal conditions of use, the air lter
4/ r
should be replaced after about 100 hours of use or one year
recommend regularly checking (10-12 applications) and replacing the air lter
it feels moist. Remove the air lter (Position
4
) replace it with a new one. Do not try to clean the lter for reuse.
The air lter must not be repaired or maintained, while it is being used by a patient.
Only use original lters! Do not use the device without a lter!
The current version of this instruction manual can be found at www
In the course of constant product improvements, we reserve the right to make
technical and design changes without prior notice.
W
In case of warranty please contact your specialist shop
or the service centre directly
please indicate the defect and attach a copy of the purchase receipt.
The following warranty conditions apply:
1.
The date of purchase is to be proven in case of warranty by the purchase receipt or invoice.
2. Defects due to material or manufacturing defects shall be repaired free of charge within the warranty period.
3.
or for the replaced components.
4. The following are excluded from the warranty:
a. any damage caused by improper handling, e.g.
by non-observance of the instruction manual.
b. Damage due to repair or intervention by the purchaser
or unauthorised third parties.
c. T
or has arisen on sending to the service point.
d.
5. Liability for direct or indirect consequential damages caused by the device
is also excluded if the damage to the device is recognised as a warranty claim.
DE/GB
GB INSTRUCTION MANUAL Inhaler IN 600/605
Legend
This instruction manual belongs to this de-
vice. The instruction manual includes impor-
tant information on the initial start-up and
handling. Read this instruction manual com-
pletely
may result in serious injury or damage to the
device.
W
These warnings must be followed to pre-
vent possible injury to the user
CAUTION
These instructions must be followed to
prevent possible injury to the device.
NOTE
These instructions provide you with useful
additional information regarding installa-
tion or operation.
Information about protection type against
foreign objects and water
Protection class II
Batch number
Manufacturer
Date of manufacture
Off/On
representative
Serial number of the device
Device and controls
Malfunctions and countermeasures
The device cannot be switched on
• Make sure the charging cable is properly plugged in/Charge the battery
• Make sure that the device has been operated within the operating period specied in
these instructions (30 min. on / 30 min. off).
The device is only misting a little or not at all
• Make sure that the air tube
7
is properly attached at both ends.
• Make sure that the air tube
7
is not compressed, bent, dirty or blocked. If necessary
replace it with a new one.
• Make sure that the nebulizer
8
is completely assembled and the atomiser head has
been correctly positioned and is not blocked.
• Make sure that the required inhalation solution is lled in the correct amount (up to 6 ml).
2
54542/54544 09/2019 V
0123
IP21
Globalcare Medical T
7th Building, 39 Middle Industrial Main Road,
European Industrial Zone, Xiaolan T
528415 Zhongshan City
PEOPLE‘S REPUBLIC OF CHINA
imported & distributed by
MEDISANA
Jagenbergstrasse 19
41468 NEUSS
GERMANY
Donawa, Lifescience Consulting Srl
Piazza Albania,
00153 Rome / Italy
EC REP
EC REP
SN
1
W
Make sure that children do not get hold of the packaging lms.
There is a risk of suffocation!
Name
Power supply
Nebulization amount (average)
Particle size
max. pressure
Noise level
Nebulizer ll quantity
Operating period
Expected service life
Operating conditions
Storage and transport conditions
Weight
Dimensions
Length of the power cable
IP class
Reference to standards
Item number
EAN number:
MEDISANAInhaler IN 600/605
Input: 100-240 V~ 50-60 Hz; Output: 5V DC, 2A
0.25 ml/min.
2.9 μm
1.1 bar
45 dBA
min. 2 ml; max. 6 ml
30 min. On / 30 min. Off
400 hours
10 - 40 °C
10 - 95 % relative maximum humidity
700 - 1060 hPa air pressure
-20 - +60 °C
10 - 95 % relative maximum humidity
700 - 1060 hPa air pressure
240 g
10.8 x 7.2 x 4.4 cm
150 cm
IP 21
EN 60601-1; EN 60601-2; 93/42/EWG
54542/54544
40 15588 54542 9/40 15588 54544 3
Aerosol properties according to EN 13544-1
Aerosol emission: 0.51 ml
Aerosol emission rate: 0.16 ml/min.
Particle size (MMAD): 2.9 μm
This device must not be disposed of with domestic waste.
All users are obligated to bring all electrical or electronic
devices to a collection point in their town/city or to a retailer
any hazardous materials or not, so that they can be disposed of in an environmentally re-
sponsible manner
or retailer with regard to disposal procedures.
T
O/I
4 5
8
9 0
7
q
6
r
w
This device meets the requirements of the Directive concerning medical devices 93/42/EEC.
Device in Class II in relation to protection against electric shocks. Nebulizer
are applied parts of type BF
e
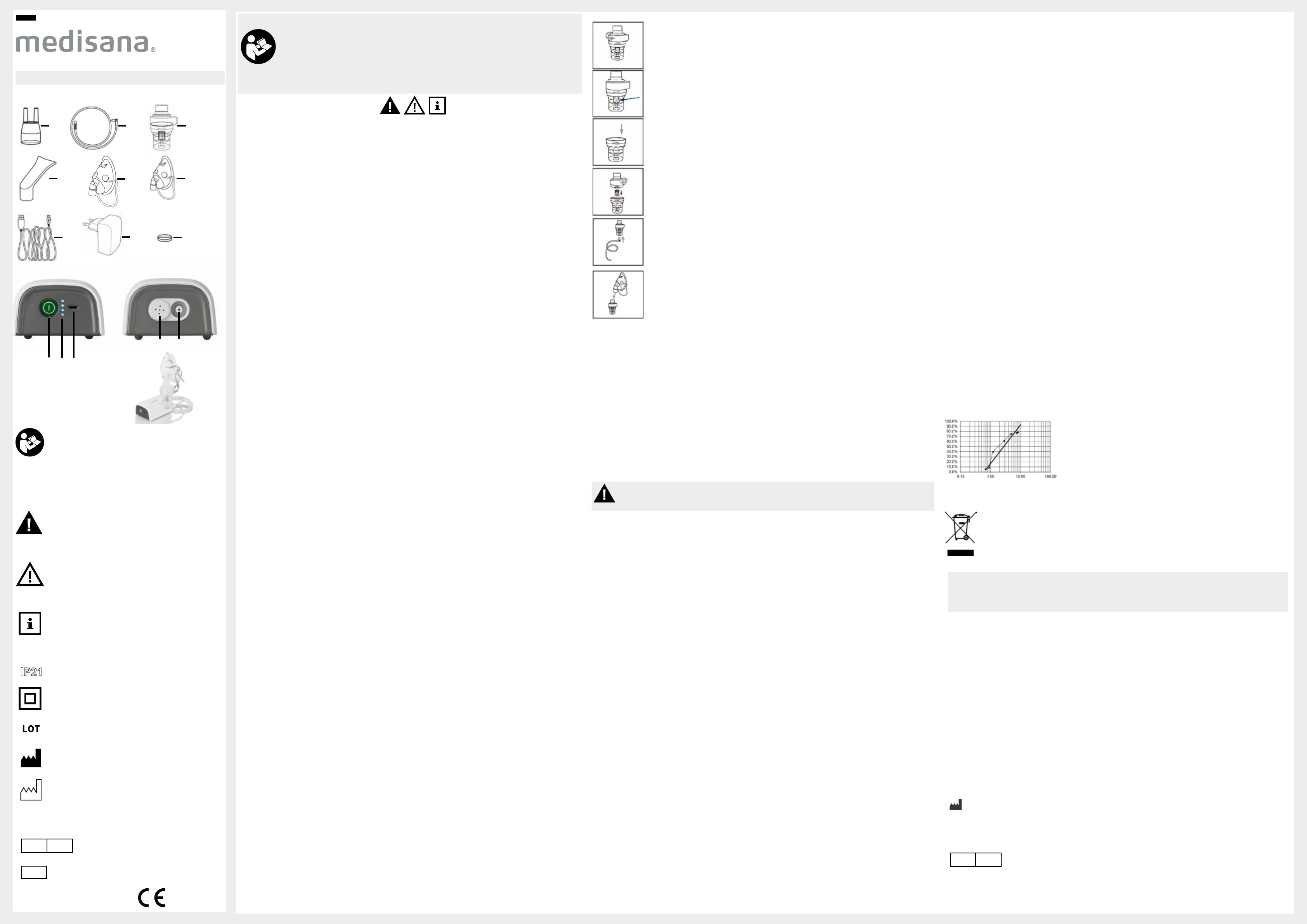
Open the nebulizer
8
by turning the cover anticlockwise.
Make
Fill the nebulizer with the inhalation solution prescribed by your doctor
maximum level (6 ml) is not exceeded.
Close the nebulizer
8
by turning the cover clockwise.
Insert the air tube
7
into the nebulizer
8
and connect the other end of the tube to the inhaler
(Position
5
).
Insert the mouthpiece or nosepiece or mask directly into the nebulizer
3